Amicus Therapeutics (FOLD) has been profiled as a bounce play from December 31th, 2012. Since then FOLD shares gained over 50% (as of now) and have continued their march to the new highs. Today Amicus Therapeutics announced positive preliminary results from all 4 dose cohorts in a Phase 2 study (Study 010) to evaluate the safety and pharmacokinetic (PK) effects of the pharmacological chaperone AT2220 (duvoglustat HCl) co-administered with enzyme replacement therapy (ERT) for Pompe disease (Myozyme(R) and Lumizyme(R)) and company’s expectations of initiating a repeat-dose clinical study in the third quarter of 2013.
John F. Crowley, Chairman and Chief Executive Officer, commented:
Study 010 has established human proof-of-concept that AT2220-ERT co-administration increases GAA enzyme activity in muscle. We look forward to initiating our repeat-dose clinical study to investigate the effect of AT2220-ERT co-administration on ERT stability and activity, ERT-related immunogenicity, and other clinical measures. We believe that co-administration may deliver significant benefits compared to ERT alone and become an important therapy for people with Pompe disease.
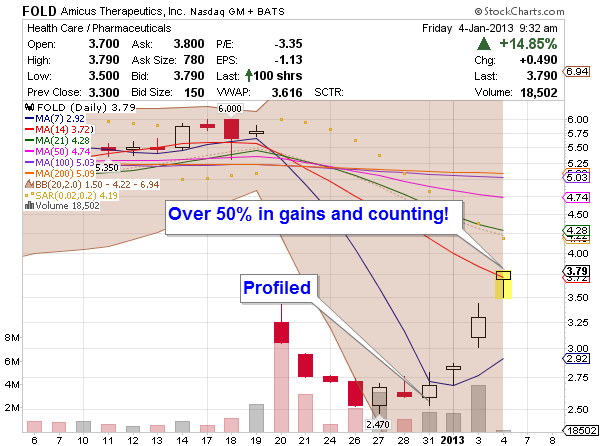
Congratulations to our followers on this trade!
Amicus Therapeutics, Inc.
1 Cedar Brook Drive
Cranbury, NJ 08512
Tel 609-662-2000
Fax 609-662-2001
Amicus Therapeutics is a biopharmaceutical company at the forefront of developing therapies for rare and orphan diseases. The Company has a robust pipeline of novel, first-in-class, small molecules called pharmacological chaperones for the treatment of lysosomal storage diseases (LSDs).

