DARA BioSciences, Inc. (DARA) announced today that company entered into an exclusive agreement with The Helsinn Group of Switzerland for U.S. commercial rights to Gelclair®.
DARA plans to launch Gelclair, FDA-cleared product indicated for the treatment of oral mucositis, in the first quarter of 2013.
Oral mucositis market is approximately 400,000 patients annually according to the research of The American Cancer Society. The National Cancer Institute estimates that almost 100 percent of patients receiving radiation therapy for head and neck cancers experience oral mucositis, as do 80 percent of patients undergoing hematopoietic stem cell transplantation and 40 percent of patients receiving standard-dose chemotherapy. DARA’s Gelclair is a topical gel used to coat and protect the oral cavity to reduce pain.
David J. Drutz, MD, DARA’s chief executive officer, commented:
The exclusive agreement with Helsinn for rights to commercialize Gelclair is a significant milestone that provides us with an important commercial product in an area of significant medical need. Hundreds of thousands of cancer patients suffer from oral mucositis each year. Helsinn offers DARA a respected partner with extensive experience in the oncology supportive care market. Our joint undertaking also provides the potential opportunity for a meaningful and long-term commercial and developmental relationship between our two companies.
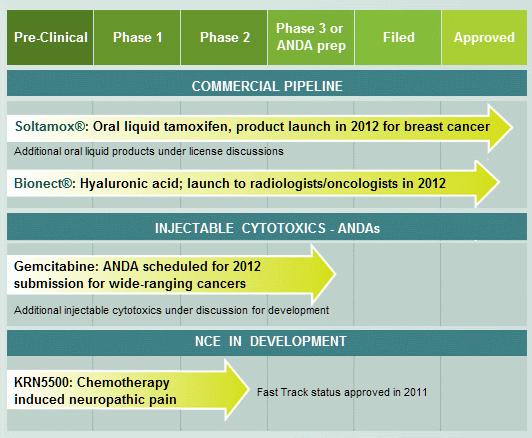
DARA BioSciences’s pipeline:
 DARA BioSciences, Inc.
DARA BioSciences, Inc.8601 Six Forks Road
Suite 160
Raleigh, NC 27615
Tel: 919-872-5578
Fax: 919-861-0239
DARA BioSciences, Inc. is a specialty pharmaceutical company focused on the development and commercialization of oncology treatment and supportive care products.
