Sientra Inc. expected to be the queen of the breast-implant industry when it entered the U.S. market in 2012 with set of new shapes and textures – so much for women to choose from.
Said so, the company was supplying about 9-10 percent of implants in the U.S., a.k.a. took roughly $30 million of $300 million breast augmentation market.
Series of unfortunate events:
- U.K.’s Medicines and Healthcare Products Regulatory Agency suspended CE certification of all of its medical devices made by Silimed.
- Sientra, Inc. (SIEN), a medical aesthetics company, today issued the following statement regarding a recent development at Silimed’s manufacturing facilities
And finally:
Sientra, Inc. (SIEN), a medical aesthetics company, today issued the following statement regarding a recent development at Silimed’s manufacturing facilities:
We are aware of a fire in one of the two manufacturing buildings at Silimed, and are in contact with Silimed in order to assess the situation. Our thoughts are with the entire Silimed team. Our management team is working diligently to get answers and we will share additional information when it is available.
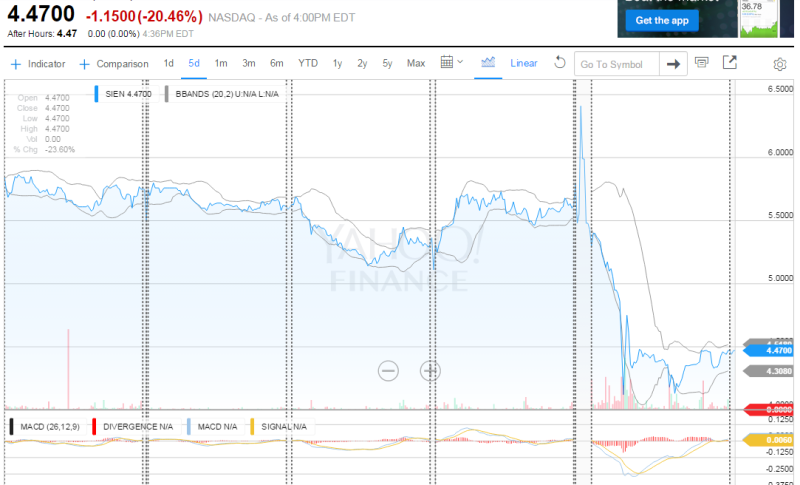
Anyhow, company stock traded much lower cash value. Unless no actual safety complains will appeared from nowhere there are multiple abilities for SIEN shares to rebound.
Disclaimer: PSH owns Sientra common shares that been bought on open market.