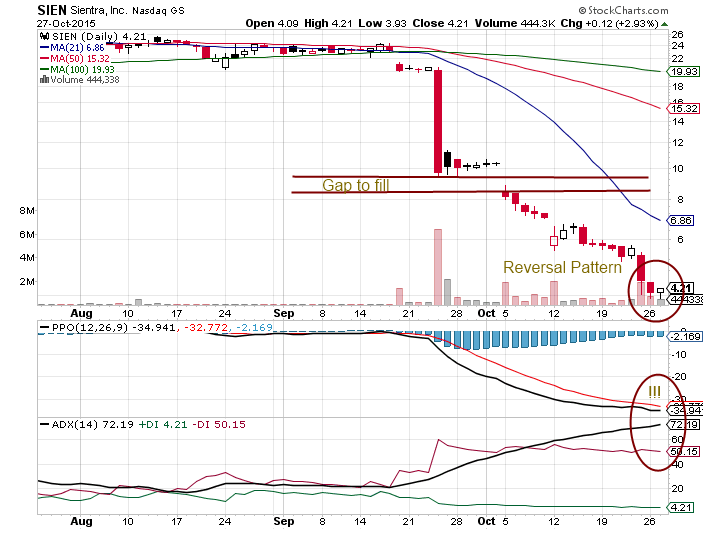
Is anyone wonder what would required from Sientra to re-open production in a new manufacturing facility?
-
Sec. 814.39 PMA supplements.
-
(a) After FDA’s approval of a PMA, an applicant shall submit a PMA supplement for review and approval by FDA before making a change affecting the safety or effectiveness of the device for which the applicant has an approved PMA, unless the change is of a type for which FDA, under paragraph (e) of this section, has advised that an alternate submission is permitted or is of a type which, under section 515(d)(6)(A) of the act and paragraph (f) of this section, does not require a PMA supplement under this paragraph. While the burden for determining whether a supplement is required is primarily on the PMA holder, changes for which an applicant shall submit a PMA supplement include, but are not limited to, the following types of changes if they affect the safety or effectiveness of the device:
-
(1) New indications for use of the device.
-
(2) Labeling changes.
-
(3) The use of a different facility or establishment to manufacture, process, or package the device.
Anyhow, take a look at chart reversal signal: